29 July 2022
New Research: Intermittent Fasting
I have always been impressed by people who subject themselves to any kind of fasting, be it long term fasting for several days, fasting intermittently on specific days each week or fasting for prolonged periods of time daily (for instance not eating from 7pm at night to 11am the following day). I remember a time in my thirties where I declared Monday as a fruit day, and only ate fruits until having a proper dinner. Even this was so difficult for me that after only a few months I stopped.
Fasting has the obvious goal and benefit of losing weight. Notably, intermittent fasting, an increasingly popular form of fasting, has been reported in humans to have additional advantages. These include improving our response to insulin, reducing inflammation, lowering our blood pressure and even inhibiting tumor growth.
Why would intermittent fasting, reportedly even without reducing our overall calorie intake, produce such beneficial effects? It is probably to a large extent due to the general phenomenon that experiencing stress makes us stronger by inducing an adaptive response.
The best example for how stress-induced adaption can exert beneficial effects is exercise. No doubt, exercise causes more damage to our joints and muscle fibers than resting, and we often feel tired and in pain after engaging in strenuous exercise. But in the long run, exercise elicits a compensatory response to repair damage, produce more muscle filaments and adapt our metabolism. This makes us stronger and more resilient when experiencing stress again.
Not all stresses seem to lead to a beneficial adaptive response. For instance, we could imagine that eating lots of junk food might lead to a response that helps us to burn fuel better and avoid the harmful effects of high glucose and lipid levels in our blood circulation. Instead, the opposite is true, eating food that contains lots of sugar and fat promotes and worsens our health, for instance by promoting the development of diabetes.
High levels of glucose and lipids in our blood induce, via a process termed glucotoxicity and lipotoxicity, the dysfunction of insulin producing pancreatic beta cells. Insulin normally lowers blood glucose, for instance by promoting glucose uptake in our peripheral organs. Consequently, lack of insulin results in elevated glucose concentrations in our blood circulation, which in turn leads to the various organ complications associated with diabetes (kidney disease, nerve damage, loss of vision etc.).
In addition to damaging our beta cells, lipids and sugar (which when in excess is converted to fat) also inhibit the response of our tissues to insulin, resulting in less glucose utilization in our muscle and liver (making these tissues “insulin-resistant”).
The fact that eating lots of fat and sugar does not lead to an adaptive response probably has to do with our evolution. For our ancestors, lack of food was a more prevalent challenge than too much unhealthy food. During starvation, fat is mobilized from our adipose tissue and lipid levels in our blood rise. Thus, high lipid levels function as a starvation signal and induce resistance of our peripheral organs to insulin. Consequently, less glucose is being used in muscle tissue or stored as fat in adipose tissue. As a result, sufficient glucose can be reserved for the brain, which is unable to utilize fatty acids and is dependent on glucose for energy production.
Another major consequence of eating junk food is the accumulation of abdominal (visceral) fat (so-called belly fat), which is a major risk factor for diabetes and cardiovascular diseases. Converting sugar, when it was available to our hunter and gatherer ancestors, into visceral fat used to be a good thing. Given that visceral fat can be quickly mobilized, it can serve as a readily available energy source when needed. This comes handy when food is only intermittently available. It is only in the modern age where constantly accessible sugar leads to the massive accumulation of belly fat.
One reason why visceral adipose tissue is so harmful is that it releases pro-inflammatory cytokines, primarily interleukin-6 (IL-6) and also TNF-alpha. These cytokines play an important role to increase insulin resistance in peripheral organs. These cytokines also induce an inflammatory state in our body. Given that inflammation is known to induce endothelial dysfunction and is also critically involved in tumorigenesis, visceral fat accumulation may also promote atherosclerosis and cancer formation.
Why visceral adipose tissue releases pro-inflammatory cytokines is harder to explain. It is believed that the amount of adipose tissue is an important signal for the nutritional status of an organisms. For instance, adipose tissue releases the hormone leptin and the production and secretion of leptin is proportional to the amount of fat tissue. When leptin levels are low, appetite is increased, and at the same time energy expenditure and energy requiring functions such as reproduction are inhibited.
Immune function and inflammation also come at a great energy cost. Hence, it appears plausible that these functions are also regulated by the amount of adipose tissue in our body, specifically by visceral adipose tissue, which changes more dynamically with nutritional status. Low amounts of visceral adipose tissue would inhibit immune function and inflammation, while abundant visceral fat promotes these processes. But there are likely other reasons why visceral adipose tissue secretes pro-inflammatory cytokines (although they seem to be not known at the present time).
Based on all this, it is not so clear why intermittent fasting would improve insulin resistance, given that under fasting conditions it would be advantageous if the peripheral organs are less sensitive to insulin and take up less glucose to preserve glucose for the brain. But the beneficial effect of intermittent fasting may be related to the fact that lower blood glucose and insulin levels during fasting periods sensitize the insulin receptors to become more responsive to insulin.
More importantly, intermittent fasting seems to have other important adaptive functions. According to an interesting website, intermittent fasting induces the production of antioxidants, promotes the repair of damaged DNA and decreases inflammation. The latter effect could be because starvation also leads to lower levels of stimulatory hormones in our blood, resulting in reduced inflammatory responses.
Finally, one very important mechanism for how intermittent fasting promotes health is the induction of autophagy, a cellular mechanism where under starvation conditions a cell digests its own macromolecules and organelles. This process takes place in organelles called lysosomes. Autophagy is the main mechanism through which cells eliminate damaged mitochondria as well as protein aggregates. This is important, because the accumulation of both damaged mitochondria and toxic protein aggregates is associated with many age-related neurodegenerative diseases.
My interest in intermittent fasting was originally prompted by a recently published research article, in which the authors looked at how intermittent fasting may be promoting weight loss and health in humans.
In the research article, the researchers studied the effects of fasting and energy deprivation in a randomized control trial. The researchers compared three fasting regiments (see figure below), 24-hour fasting with 150% calorie intake on alternate days, continuously reduced calorie intake (75% daily calorie intake, corresponding to the same total energy intake as the first group), fasting without net calorie restriction (alternate days of fasting and 200% energy intake) and a control group with matched calorie intake (daily 100% energy intake). These regimens were maintained for 3 weeks followed by measurements of body mass and composition as well as a number of other measures of cardiovascular health.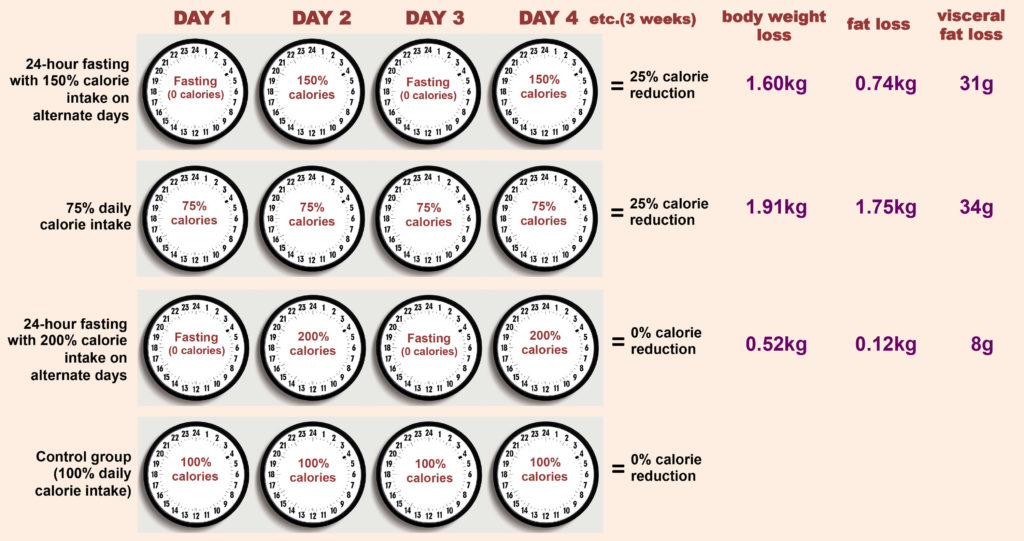

In this recent study, Ulgheralt and co-workers investigated the effects of overnight intermittent fasting. The authors used Drosophila, i.e. fruit flies, as a model organism. They did not actually look at effects on weight, but at effects on lifespan and the so-called health span (the time period in our lives up to which we remain healthy).
In their study, the researchers subjected flies to a regimen of 20-hour fasting periods that included the night (when fruit flies, like humans, are least active). The fasting for 20 hours was alternated with feeding periods of 28 hours. The researchers chose this specific schedule of 20 hours of fasting and 28 hours of feeding because it produced to the most robust extension in lifespan in the flies.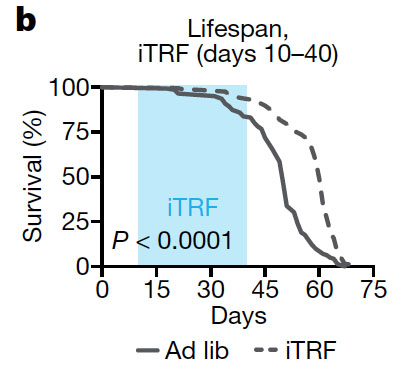
Intermittent time-restricted feeding (iTRF) increased the lifespan (% survival over time) compared to flies with free access to food (Ad lib). The intermittent fasting regimen was implemented for 30 days between day 10 to day 40 of the flies’ lifetime (blue area in the graph).
The 20 hour fasting on alternate days increased lifespan by 18% in females and by 13% in males compared with flies with unrestricted access to food. Importantly, when the researchers shifted the 20 hour fasting period by 12 hours, so that the fasting period occurred mostly during the day instead of at night, the life extension effect was lost. This suggests that the beneficial effects of intermittent fasting are dependent on extended food deprivation including the nighttime, which is the commonly suggested intermittent fasting regimen in humans.
Interestingly, the intermittent fasting did not lead to an overall decreased, but actually an increased calorie intake (meaning that the flies overcompensated by eating more during the feeding periods). These results indicate that the beneficial health effects of intermittent fasting are not dependent on eating less calories, at least in flies. That said, in order to lose weight with intermittent fasting, an overall calorie reduction is probably necessary, as shown in the human study.
It is also noteworthy that the intermittent fasting did not only increase lifespan, but also the health span of the flies. Thus, intermittent fasting improved muscle function, which in the flies was detected as less age-related decline in climbing ability relative to flies on a normal diet. Intermittent fasting also protected against a decline in neuronal function, which is important, given that one of the major group of diseases associated with aging are neurodegenerative disorders.
Age-related neurodegenerative diseases have been shown to be associated with and be at least partially due to the formation of protein aggregates. To measure the amount of protein aggregates, the authors extracted detergent-insoluble proteins from aged flies. They found a decrease in protein aggregates in flies undergoing intermittent fasting. Given the involvement of protein aggregation in neurodegenerative disease, this result suggests that fasting intermittently may help us to avoid age-related diseases such as Alzheimer’s and Parkinson’s Disease for longer.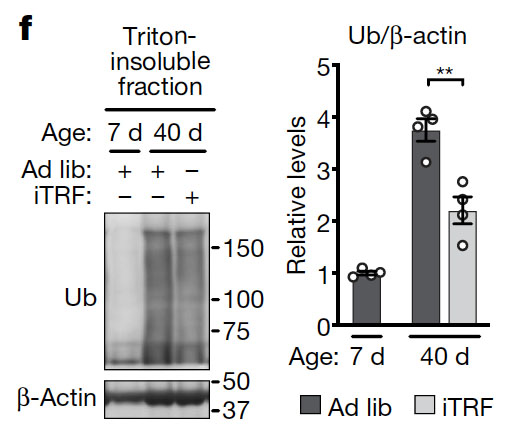
Protein aggregates are usually detergent insoluble and modified with the small polypeptide ubiquitin. Hence, to measure protein aggregates, the researchers prepared the detergent (triton)-insoluble protein fraction of flies and analyzed this fraction by Western blotting using a ubiquitin (Ub) antibody. Remarkably, there was a huge increase in protein aggregates from day 5 to day 40. Intermittent time-restricted feeding (iTRF) reduced the amount of protein aggregates compared to flies with free access to food (Ad lib). The bar graph on the right shows the average of four independent experiments.
What is the mechanism through which intermittent fasting reduces cellular protein aggregates?
Degradation of protein aggregates normally involves a process called autophagy. Autophagy literally translates into “self eating”, which means that cells digest parts of themselves (parts of the cytosol, organelles, and protein aggregates) and break these parts down into small molecules such as amino acids and sugars. Autophagy is important during times of starvation, where the breakdown of cellular components helps the cell to generate fuels for energy production and building blocks for the synthesis of essential macromolecules. Autophagy is also important to eliminate damaged organelles and toxic protein aggregates.
What the researchers found in the study is that intermittent fasting increases autophagy activity. What is more, knocking down the expression of critical autophagy genes prevented lifespan extension induced by the intermittent fasting.
Why is it important that the calorie reduction includes the night period?
This may be partly related to the fact that autophagy occurs mostly during the nighttime. For instance, the expression of critical autophagy genes peaks at night. However, it is not the increased expression level of autophagy genes alone that is responsible for the beneficial of nighttime intermittent fasting. Thus, the authors have shown that nighttime specific overexpression of critical autophagy genes mimics the effect of intermittent fasting on lifespan extension, while daytime specific overexpression does not. This means that likely there are other factors involved.
Of all the results in the paper, the discovery that overexpression of autophagy genes (during nighttime) can actually extend the lifespan is the most exciting finding for me. It suggests that by only activating autophagy, we can live longer!
The study also suggests that one reason why getting more sleep (by potentially activating autophagy?) may be important is because it can prevent neurodegenerative diseases. This is something I have been concerned with for some time. It has prompted me to make a major change in my own life by consistently getting more sleep.