Lab Research
Our lab focusses on how metabolic changes are involved in human diseases and ageing, and how we can manipulate cell metabolism and cell signalling to come up with novel therapeutic strategies.
Some of our recent (and partly still ongoing) research projects are highlighted below:
WHAT MAKES US AGE?
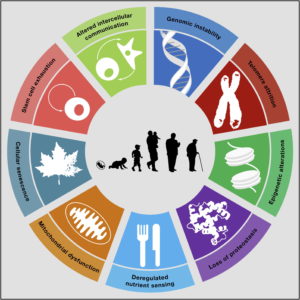
This is a question that many of you will have asked yourself at one point. There are in fact many theories of ageing. One of the most popular ones is the Free Radical Theory of Ageing (a.k.a. FRTA). It appears to be a plausible theory, according to which ageing is due to cumulative oxidative damage to our cells and organs, resulting from constant reactive oxygen species (ROS) production. The major source of cellular ROS is the mitochondrial electron transport chain. The generated ROS, such as superoxide, hydrogen peroxide and hydroxyl radicals, can react with proteins, DNA and lipids, leading to protein dysfunction, DNA mutations and membrane damage, respectively. Due to the high reactivity of ROS, most of the damage incurred by ROS is expected to be directly in the mitochondria. Indeed, mitochondrial dysfunction with ageing has been consistently observed in various ageing model system. Mitochondrial dysfunction has in fact been recognized as one of the hallmarks of ageing (see Figure).
 From: Carlos Lopez-Otin et al. The Hallmarks of Ageing. (2013) Cell Volume 153, Issue 6, p.1194-1217.
From: Carlos Lopez-Otin et al. The Hallmarks of Ageing. (2013) Cell Volume 153, Issue 6, p.1194-1217.
The fact that there is mitochondrial dysfunction with ageing is of course not proof that the FRTA is indeed true. Providing proof would require to demonstrate that mitochondrial dysfunction during ageing and ageing itself is actually due to ROS, or in other words to show that preventing oxidative damage prevents mitochondrial dysfunction and extends lifespan. To provide this proof, researchers have carried out intervention experiments in various model organisms. In these studies, the researchers used treatment with antioxidant compounds or transgenic animal models with altered expression of antioxidant enzymes to prevent oxidative damage. However, these experiments have often not resulted in the expected results and generally not resulted in an extension of lifespan. This has resulted in wide-spread scepticism about the validity of the FRTA.
If not ROS, what then is the cause of mitochondrial dysfunction during ageing? Apart from oxidation, another form of protein modification is acylation. Protein acylation is the addition of an acyl group to a protein, typically to the ε-amino group of lysine amino acids in proteins (see Figure). The substrates for protein acylation are energy rich acyl-coenzyme A thioesters, such as acetyl-CoA and succinyl-CoA, resulting in protein acetylation and succinylation, respectively. The best-known protein substrates that become acylated with an acetyl group are histone proteins. Histone acetylation plays an important role in regulating gene expression and is catalysed by specific histone acetyl transferases. It has become clear in recent years that numerous mitochondrial proteins are also acylated (Wagner and Payne, 2013). In contrast to histones, mitochondrial proteins are acylated in a non-enzymatic manner. This chemical process is aided by the high mitochondrial acyl-CoA concentrations and the higher pH found in mitochondria (Wagner and Payne, 2013).
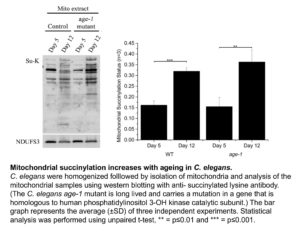
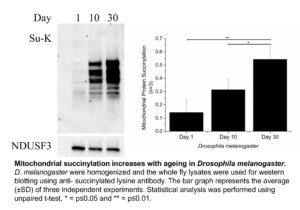
Of note, acylation of mitochondrial proteins normally leads to an inhibition of the function of the modified protein. Based on this, we hypothesised that mitochondrial dysfunction during ageing could be a consequence of an increase in mitochondrial protein acylation. A former PhD student in the lab, Hong Shin Yee, took a first step to test this hypothesis, by trying to test if there is an age-dependent increase in mitochondrial protein acylation.
Shin Yee looked at three different organisms, the roundworm C. elegans, Drosophila melanogaster (fruit fly) and mice. We did not find a robust increase in protein acetylation with increasing age. However, Shin Yee observed that mitochondrial protein succinylation increases with age in both C. elegans and Drosophila (see Figure). This result raises the possibility that an age dependent increase in protein succinylation could be a contributing factor to mitochondrial dysfunction during ageing.
However, when Shin Yee looked for age dependent changes in protein succinylation in mice, no consistent trends could be found. What could be the reason for the discordant results in C. elegans and Drosphila on the one hand versus mice on the other hand. There are a number of possible explanations. For instance, the mice that we studied may simply not have been old enough to display an increase in mitochondrial protein succinylation. There is also another possibility, which is that the age dependent increase in protein succinylation in C. elegans and Drosophila is due to the absence of deacylating enzymes (known as Sirtuins) in these organisms.
Sirtuins are proteins that can remove acyl groups, such as acetyl- and succinyl-modifications, from proteins. Mice express seven sirtuin isoforms (SIRT1-SIRT7). Three of these sirtuin enzymes (SIRT3, SIRT4, and SIRT5) are localized in mitochondria. C. elegans and Drosophila also have multiple SIRT isoforms, but the only isoform that is present in mitochondria is a homolog of SIRT4. Of note, SIRT5, the major SIRT isoform that removes succinyl groups from proteins, is absent in both C. elegans and Drosophila. Hence, this suggests that the presence of SIRT5 in mice (and humans) may be important to prevent an age-dependent increase in mitochondrial protein succinylation and maybe even to protect ourselves from mitochondrial dysfunction during ageing. As always, more research is required to test these hypotheses… For now, we can say that in addition to protein oxidation, protein acylation is another protein modification that could be of importance in ageing and human disease.
To read more about this exciting topic, you can click on this link, which will take you to a student assignment of one of the former LSM2103 (now LSM2233) Cell Biology students. www.sibiol.org.sg/sites/default/files/AYJB/AYJB_2014v02_21-23.pdf
Other references:
Hong SY, Ng LT, Ng LF, Inoue T, Tolwinski NS, Hagen T, Gruber J. The Role of Mitochondrial Non-Enzymatic Protein Acylation in Ageing. PLoS One (2016) Volume 11, p.e0168752 www.ncbi.nlm.nih.gov/pubmed/?term=28033361
Wagner GR, Payne RM. Widespread and enzyme-independent Nε-acetylation and Nε-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J. Biol. Chem. (2013) Volume 288, pp.29036-45 www.ncbi.nlm.nih.gov/pubmed/23946487
Carlos Lopez-Otin et al. The Hallmarks of Ageing. Cell (2013) Volume 153, p.1194-1217 www.ncbi.nlm.nih.gov/pubmed/23746838
HOW DO CANCER CELLS INDUCE THE WARBURG EFFECT?
The Warburg effect of cancer cell metabolism was discovered by Otto Warburg about a hundred years ago. And researchers are still studying its mechanism and role in tumorigenesis. That is amazing.
What is the Warburg effect? The Warburg effect is the characteristic of cancer cells to predominantly produce their energy through a high rate of glycolysis followed by lactic acid production. In other words, cancer cells predominantly produce ATP from glycolysis and not like other cells mostly from oxidative phosphorylation in mitochondria, even in the presence of abundant oxygen. It is believed that one reason why cancer cells undergo the Warburg effect is that it allows them to have more metabolic intermediates available as building blocks for the synthesis of macromolecules. These intermediates include metabolites derived from the TCA cycle due to lower oxidative phosphorylation rates and from glycolysis due to greater glucose intake. Synthesis of macromolecules from these building blocks is essential to support tumor cell growth and division.
 Otto Warburg, the discoverer of the “Warburg effect”
Otto Warburg, the discoverer of the “Warburg effect”
How do cancer cells re-program their metabolism to adopt the Warburg like phenotype? For a Warburg like metabolism to be induced, there are two major changes that must take place. Firstly, there is inhibition of oxidative phosphorylation in the mitochondria. This is frequently achieved by preventing pyruvate metabolism and diverting the pyruvate from mitochondria to being converted to lactate in the cytosol. Pyruvate diversion is commonly induced through inhibition of pyruvate dehydrogenase via phosphorylation. Pyruvate Dehydrogenase normally converts pyruvate in mitochondria to acetyl-CoA, which in turn then enters the TCA cycle. Another mechanism through which mitochondrial pyruvate metabolism is inhibited is via a downregulation of the mitochondrial pyruvate transporter in cancer cells. As a result, uptake of pyruvate into mitochondria is inhibited.
In the absence of oxidative phosphorylation, cells need to produce ATP via glycolysis. However, glycolysis only produces a fraction of the ATP that is normally produced by oxidative phosphorylation. Hence, cells must greatly increase their glucose consumption and glycolytic rate. Therefore, the second requirement for the induction of the Warburg effect is an upregulation of glycolysis. However, the mechanisms involved in the upregulation of glycolysis are less well understood.
One of the well-known activators of glycolysis is insulin, especially in insulin sensitive tissues such as skeletal muscle and adipose tissue. In these tissues, insulin functions primarily by promoting the translocation of the glucose transporter GLUT4 from intracellular storage vesicles to the plasma membrane. Signaling by growth factors such as insulin is frequently also activated in cancer, often as a result of activating mutations in Receptor Tyrosine Kinases or downstream intermediates in the insulin signaling pathway. However, cancer cells do not express GLUT4.
Of note, there is evidence that in cancer cells insulin signaling regulates a different glucose transporter, GLUT1. For instance, it has been shown that inhibiting insulin signaling in a cancer cell line with a constitutively active insulin signaling pathway prevents GLUT1 plasma membrane localisation (Makinoshima et al., 2015). However, what is the mechanism through which insulin signaling regulates GLUT1 plasma membrane localization?
Enters TXNIP, or Thioredoxin Interacting Protein. As the name suggests, TXNIP was originally found to regulate the activity of the antioxidant protein thioredoxin. However, when TXNIP knockout mice were generated some 10 years ago, it became clear that the major function of TXNIP is to regulate glucose and lipid metabolism. One of the major phenotypes of the TXNIP knockout mice is a low blood sugar concentration (or hypoglycemia). TXNIP was in fact found to normally inhibit cellular glucose utilization. Hence, lack of TXNIP expression leads an increase in cellular glucose consumption and consequently to hypoglycemia.
How does TXNIP inhibit cellular glucose utilization? TXNIP actually promotes the endocytosis of the GLUT1 glucose transporter (Wu et al., 2013). As a result, TXNIP downregulates the cellular uptake of glucose.
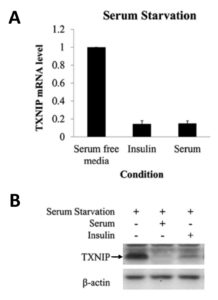
Based on the roles of insulin and TXNIP to regulate cellular glucose uptake via GLUT1, we hypothesized that insulin signaling might increase cellular glucose uptake by downregulating TXNIP expression. As shown in the Figure below, we found that this is indeed the case. Addition to cells of serum (which contains various growth factors that activate insulin signaling) or of insulin itself leads to a marked downregulation of TXNIP mRNA and protein expression. Conversely, inhibiting insulin signaling led to marked upregulation of TXNIP. Of note, it has been reported that TXNIP is downregulated in tumors and that lack of TXNIP promotes the development of various cancers. In conclusion, downregulation of TXNIP upon activation of insulin signaling is likely important in increasing glycolysis in cancer cells and thus promoting the Warburg effect.
Serum and insulin suppress TXNIP expression at the protein and mRNA levels.
HeLa cells were starved of serum (containing growth factors) overnight, followed by the addition of insulin or 10% fetal bovine serum for 4 hours. The mRNA level of TXNIP was quantified using quantitative Real-Time-PCR (A) or by Western blotting (B).
Here is the link to Shin Yee’s paper, in which this work is described in detail.
Hong SY, Yu FX, Yan L, Hagen T. Oncogenic activation of the PI3K/Akt pathway promotes cellular glucose uptake by downregulating the expression of thioredoxin-interacting protein. Cellular Signalling (2016) 28:377–383 ncbi.nlm.nih.gov/pubmed/26826652
Other references:
Makinoshima H, Takita M, Saruwatari K, Umemura S, Obata Y, Ishii G, et al. Signaling through the phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) axis is responsible for aerobic glycolysis mediated by glucose transporter in epidermal growth factor receptor (EGFR)-mutated lung adenocarcinoma. J Biol Chem (2015) 290:17495-17504 www.ncbi.nlm.nih.gov/pubmed/26023239
Wu N, Zheng B, Shaywitz A, Dagon Y, Tower C, Bellinger G, et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell (2013) 49:1167–1175 www.ncbi.nlm.nih.gov/pubmed/23453806
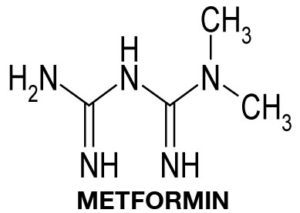
MECHANISM OF ACTION OF THE ANTI-DIABETES DRUG METFORMIN
If you have read the post about the Warburg effect in cancer cells, you already know that we are interested in the TXNIP protein. To summarize again what TXNIP is, the abbreviation stands for Thioredoxin Interacting Protein. The main function of TXNIP appears to be the inhibition of cellular glucose metabolism. TXNIP inhibits plasma membrane localisation of GLUT1, the major glucose transporter in most cells. As a result, cellular glucose uptake is inhibited. Recent evidence suggests that TXNIP also negatively regulates the insulin-sensitive GLUT4 glucose transporter.
Metformin is the most commonly prescribed drug for the treatment of diabetes. Diabetes starts with the development of insulin resistance in organs such as skeletal muscle, adipose tissue and liver. Insulin resistance is initially compensated by increased insulin secretion by pancreatic beta-cells. However, eventually beta-cells fail to produce the required amounts of insulin, leading to a rise in the blood glucose concentration and the development of full Diabetes. Metformin functions by improving insulin resistance, in other words by increasing cellular uptake and utilisation of glucose.
The mechanisms through metformin exerts its effect to increase cellular glucose localization are still being debated. One proposed mechanism is the inhibition of oxidative phosphorylation in mitochondria. This effect of metformin is due to inhibition of complex I of the electron transport chain and/or the enzyme glycerol-3-phosphate dehydrogenase 2, which delivers electrons to coenzyme Q in the electron transport chain. Inhibition of the mitochondrial electron transport chain leads to lower rates of ATP synthesis in the mitochondria. As a result, the cells are forced to produce more ATP via glycolysis. However, the yield of ATP produced from glycolysis is much lower compared to ATP production from mitochondrial oxidative phosphorylation. As a result, the cells need to take up more glucose, thus improving glucose disposal and insulin sensitivity.
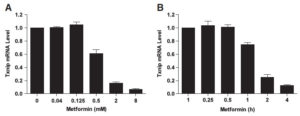
We hypothesised that metformin may also have a direct effect on cellular glucose uptake by regulating the expression of TXNIP. This idea came originally from the work of our collaborator Dr. Faxing Yu from IMCB, A*STAR (who is now a professor at Fudan University). Faxing showed that metformin dramatically downregulates TXNIP expression in different cell lines. Tiffany Chai, an honours student in our laboratory, then studied this effect.
Txnip protein and mRNA expression are downregulated in the presence of metformin. HeLa cells were treated with different concentrations of metformin for 2 h (A) or 2 mM metformin for various periods of time (B), followed by measuring the Txnip mRNA expression with quantitative Real-Time-PCR.
The main regulator of TXNIP expression is the MondoA/MLX transcription factor. Tiffany showed that metformin reduces the binding of MondoA/MLX to the TXNIP promoter. But how does metformin regulate the MondoA/MLX transcription factor?
MondoA/MLX activity is dependent on glucose. As mentioned above, metformin is known to inhibit mitochondrial electron transport chain activity and consequently to increase cellular glucose uptake and utilisation via glycolysis. As expected, treatment of cells with metformin resulted in a lower consumption of oxygen, the terminal electron acceptor of the electron transport chain. Upon metformin treatment of cells, we also observed a greater consumption of glucose and an increased conversion of glucose into lactate.
We therefore wanted to know if the increased glycolytic rate induced by metformin plays a role in the repression of TXNIP expression by the anti-diabetes drug. Thus, we tried to prevent the increase in the glycolytic rate upon metformin addition with the GAPDH inhibitor iodoacetate. Interestingly, we found that iodoacetate prevented both the effect of metformin on glycolysis and on TXNIP expression. Similarly, siRNA mediated silencing of the expression of the glycolytic enzyme aldolase also prevented the metformin induced decrease in TXNIP expression. This suggests that the effect of metformin on TXNIP expression is related to the increased glycolytic rate upon drug treatment. However, the exact mechanism through which glycolytic flux regulates TXNIP expression remains to be elucidated.
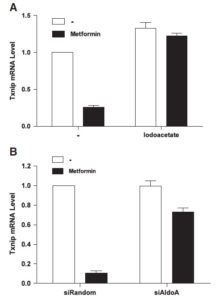 Inhibition of GAPDH or Aldolase enzyme activity prevents the TXNIP downregulation induced by metformin. (A) HeLa cells were preincubated with 0.5 mM iodoacetate for 10 min followed by 4 h incubation in the presence of 2 mM metformin. Txnip mRNA level was measured using quantitative Real-Time-PCR. (B) Aldolase expression was suppressed using aldolase siRNA. After incubation for 3 days, 2 mM of metformin was added and cells were incubated for 4 h followed by mRNA extraction and quantitative RT-PCR.
Inhibition of GAPDH or Aldolase enzyme activity prevents the TXNIP downregulation induced by metformin. (A) HeLa cells were preincubated with 0.5 mM iodoacetate for 10 min followed by 4 h incubation in the presence of 2 mM metformin. Txnip mRNA level was measured using quantitative Real-Time-PCR. (B) Aldolase expression was suppressed using aldolase siRNA. After incubation for 3 days, 2 mM of metformin was added and cells were incubated for 4 h followed by mRNA extraction and quantitative RT-PCR.
Here is Tiffany’s paper:
Chai TF, Hong SY, He H, Zheng L, Hagen T, Yan L, Yu FX. A potential mechanism of metformin-mediated regulation of glucose homeostasis: Inhibition of Thioredoxin-interacting protein (Txnip) gene expression. Cellular Signalling (2012) 24:1700–1705
www.ncbi.nlm.nih.gov/pubmed/23453806