29 June 2023
The carbohydrate-insulin model
One of the my main problems when going for running training used to be becoming hypoglycemic and dizzy during the first 15 min. Hence, I usually started my workouts by exhausting myself partially and subsequently taking a rest to let the blood sugar level go up again. Only then I started with my real exercise.
It took me a long time to figure out the reason – my false belief that going hungry into a run would compromise my running ability. This false assumption led me to always ensure I ate something before running. However, through trial and error I realized at some point that I can actually run perfectly well without having eaten for a few hours. Most importantly, by not eating before going for my running trainings I also avoid getting dizzy.
The simple explanation for this phenomenon is the meal-induced insulin release. The released insulin lowers the blood glucose level via a number of mechanisms. These include inhibiting new glucose production in the liver as well as inhibiting breakdown of stored glucose (in the form of glycogen) and release of glucose from the liver. Insulin also promotes the uptake of glucose into fat tissue where glucose is converted into fat. Finally, insulin inhibits the release of free fatty acids from adipose tissue and instructs muscle tissue to use glucose instead of fatty acids.
In short, insulin promotes glucose and fat storage and inhibits glucose and fatty acid release into the blood. If I start to exercise under these conditions and try to utilize glucose and fatty acids, it is no surprise that I run out of energy reserves and become hypoglycemic and dizzy.
Notably, the same mechanism forms the basis for a major hypothesis to explain the obesity pandemic observed worldwide over recent decades – the so-called carbohydrate-insulin model.
According to this model, the culprit of the obesity endemic is the abundance of palatable food rich in carbohydrates. Absorption of food high in carbohydrates (= food with a high glycemic load) rapidly increases the blood glucose concentration. This in turn stimulates the release of glucose-lowering insulin from the pancreas. At the same time there is a drop in the levels of another hormone, glucagon, which normally increases the blood glucose concentration.
High insulin levels and a high insulin-to-glucagon ratio have two obesity-relevant effects. Firstly, insulin promotes energy storage in fat tissue and suppresses the release of fatty acids from our fat depots, thus promoting weight gain.
Secondly, elevated insulin concentrations and a high insulin-to-glucagon ratio in our blood lead to a rapid drop in the blood glucose concentration to values even lower than the initial concentration. As a result, the individual experiences a deficiency of energy-containing substrates in the blood (via reduced levels of glucose and fatty acids) some time (around 3 to 5 hours) after consuming the carbohydrate-rich meal. According to the carbohydrate-insulin model, the energy deficiency in the blood is sensed by the brain,making us crave for food again. In addition, upon sensing of a low energy state, the brain also reacts by reducing our energy expenditure, further promoting weight gain.
The model suggests that fat accumulation is not primarily due to eating too much. Instead, because the carbohydrate-rich food we consume is deposited in adipose tissue and not used for energy production, we eat more to compensate for this state of energy deficiency. Jeff Flier, in his excellent commentary article recently published in Science, refers to the carbohydrate-insulin model as being a “pull theory” of energy deficiency (the body is pulling in more food due to the perceived energy deficiency), as opposed to the traditional “push” theory of overeating.
It is important to realize that the carbohydrate-insulin model is only a model, which is far from proven. However, Jeff Flier cites an important study by Shimy et al., published in 2020, in support of the model.
Shimy et al. studied 29 adults (aged 20 to 65 years) with overweight or obesity (body mass index ≥ 25 kg/m2). Before participating in the study, the subjects had achieved considerable weight loss (10% to 14% of their body weight). During the following weight maintenance period, the participants were then randomly divided to consume three different test diets varying in carbohydrate content:
-a high-carbohydrate diet (with 60% of total energy content derived from carbohydrates),
-a moderate carbohydrate(with 40% of total energy content derived from carbohydrates), and
-a low-carbohydrate diet(with 20% of total energy content derived from carbohydrates).
The total energy content and protein content (20% of the energy content) were kept constant. During week 10 and 15 on the test diets, laboratory studies were conducted to measure metabolic fuels and hormones concentrations in the blood.
What did the study find?
The hormone measurements showed that the insulin concentration (measured as the area under the curve during the first 30 to 120 minutes after ingesting a meal) was indeed higher in the high carbohydrate group compared to the moderate and low carbohydrate groups. As expected, the trend was the opposite for glucagon. Remarkably, the insulin-to-glucagon ratio was 7-fold higher in participants on the high versus the low carbohydrate diet.
What about the energy availability (measured as the blood concentrations of energy-providing metabolites)?
The study found that the total early postprandial (30 to 180 minutes after meal ingestion) energy availability declined fastest in the high-carbohydrate group. The late postprandial (180 to 300 minutes after meal ingestion) energy availability was markedly lower in the high carbohydrate group (see figure below). These findings are consistent with the carbohydrate-insulin model.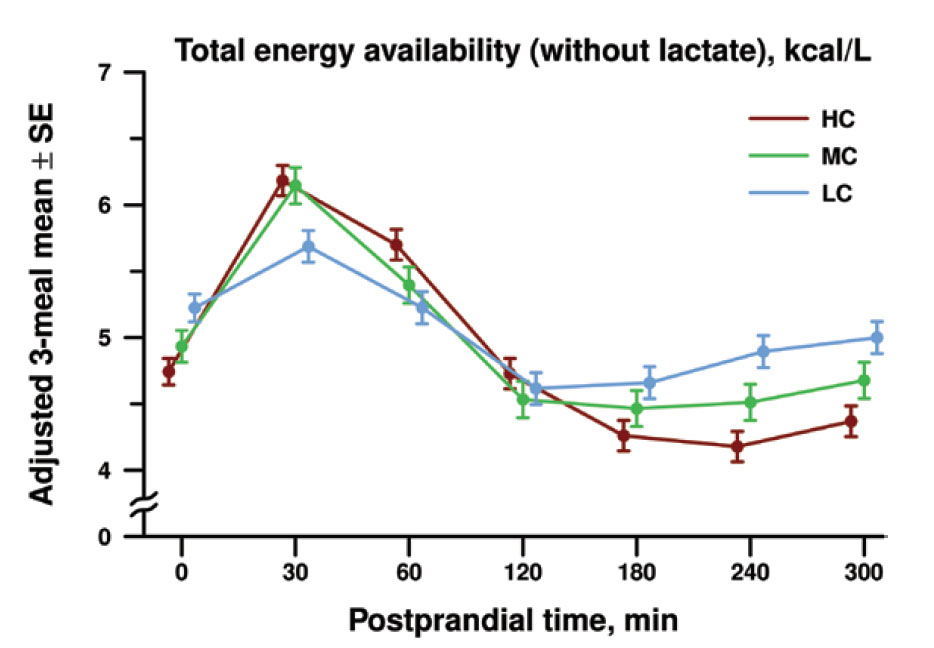
Postprandial total energy availability of metabolic fuels (including glucose, ketone bodies and free fatty acids). The authors combined the postprandial energy availability for all 3 meals (dinner, breakfast, and lunch) to calculate a diet-specific mean for high carbohydrate (HC), moderate carbohydrate (MC), and low carbohydrate (LC) diets at each postprandial time point in minutes. The late postprandial energy availability was 0.58 kcal/L lower on the high versus the low carbohydrate diet.
The authors then wanted to determine which metabolites accounted for the decreased energy availability in the high carbohydrate group. As discussed, insulin lowers blood glucose levels. However, the researchers found that the decreased energy availability in the high carbohydrate diet group was not due to lower blood concentrations of glucose. Instead, subjects on the high carbohydrate diet had lower concentrations of two other important fuels, free fatty acids and ketone bodies. Insulin is known to inhibit the release of free fatty acids from adipose tissue and to also strongly inhibit the synthesis of ketone bodies, a type of fuel that is used by the body under conditions of energy starvation.
The important question then is how would a lower energy availability make us more hungry and what are the signals our brain senses to make us crave for food?
There are in fact several inputs into the brain to signal our fullness and energy availability.
We have both hunger-causing nerve cells and as well as hunger-inhibiting nerve cells in the hypothalamus in our brain. These nerve cells can directly sense blood glucose levels.
Our body also releases hormones that can regulate the activity of hunger sensing neurons. For instance, the hormone ghrelin is produced by endocrine cells in the stomach under conditions when the stomach is empty. Ghrelin increases the activity of the hunger-causing nerve cells and reduces the activity of hunger-inhibiting cells.
In addition, there are hormones, such as Peptide YY and glucagon-like peptide 1 (GLP-1), that are released from the intestine in response to food in the gut and that make us feel full. Notably, recently developed GLP-1 mimicking drugs (or GLP-1 receptor agonists) have shown remarkable weight loss effects in initial human trials and have emerged as the most promising and effective anti-obesity medications that have thus far been developed.
Finally, another important appetite regulating hormone is leptin, which is produced by adipose tissue as a function of the fat content and then released into the blood. Leptin causes the brain to lower our appetite and increase our metabolic activity (or our energy expenditure). Hence, leptin is an important weight loss-promoting hormone.
Leptin is also acutely regulated. For instance, fasting dramatically decreases leptin levels, whereas refeeding restores them. Fasting is accompanied with increased blood levels of adrenergic hormones (norepinephrine, epinephrine), which have been shown to inhibit leptin release.
However, none of the described mechanisms is likely to be different between the high and low carbohydrate groups. The stomach and intestinal filling would be expected to be similar. Furthermore, the authors found no differences in blood energy availability derived from glucose as well as no differences in adrenergic hormone levels.
There are other proposed hunger and fullness sensing mechanisms, Some (but not all) research studies suggest that insulin (which IS elevated in the low carbohydrate compared to the high carbohydrate diet group) promotes leptin secretion. However, this would mean that a high glycemic load would, via increased insulin levels, decrease hunger, which goes contrary to the carbohydrate-insulin model.
On the other hand, leptin release from adipose tissue has also been reported to be stimulated by short chain fatty acids. A study published in 2004 reported that short-chain fatty acids with a length of 2 to 6 carbon atoms stimulate leptin expression via binding to the orphan G protein-coupled receptor GPR41 at the cell membrane. Of note, short-chain fatty acids are produced in large amounts in the lower intestine through fermentation of dietary fibers, which are expected to be more abundant in low carbohydrate diets.
Did the researchers find any differences in hunger and appetite between the different groups?
Surprisingly, the study found no significant differences in hunger scores between the three test diet groups. The hunger scores were obtained at each time blood was drawn and were based on ratings by the study participants in response to the question “How hungry are you right now [on a scale of 1 to 10]?”.
Even more surprisingly, the satiety scores (based on participants ratings in response to the question “How full are you right now?”) were lower in the low carbohydrate compared to the high-carbohydrate group during the early and late postprandial periods. This means that although subjects consuming the carbohydrate rich diet had lower blood energy levels, they felt equally hungry and more full compared to those on the low carbohydrate diet.
The authors discussed a number of potential explanations. For instance, self-reported scales may not reliably predict hunger and fullness. The authors also pointed out that the survey questions did not distinguish between the two types of hunger, homeostatic hunger (the motivation to eat due to declining energy stores) and hedonic hunger (desire to eat palatable foods). This is important because only homeostatic hunger would be expected to directly correlate with energy availability.
Nonetheless, the unexpected hunger scores pose a question mark about whether the carbohydrate-insulin model can truly explain the increase in food consumption in our modern society.
If I consider my own experience with reducing the carbohydrate content in my food, I find that my satiety level depends mainly on the food volume I eat, and less on the actual calorie content. For instance, I find eating soup more filling than dry food. I also do find that eating carbohydrate rich food makes me crave for more carbohydrate rich food, both early on and during later times after a meal.
Coming back to the paper by Shimy et al., it can be concluded that their study provides some support for the carbohydrate-insulin model of obesity, where an elevated insulin-to-glucagon ratio in response to a high-carbohydrate diet directs metabolic fuels toward storage, resulting in fat gain and in lower circulating energy. The lower energy availability in our blood after consuming a carbohydrate rich meal may make us more hungry, although there are question marks about this conclusion. The results suggest that an alternative strategy to fight obesity is to reduce the total carbohydrate or specifically the high-glycemic index carbohydrate intake.
However, the carbohydrate-insulin model of obesity is still far from proven. As Jeff Flier points out, the model may contribute to obesity in a subset of the obese population, for instance in those individuals who respond to a standard load of glucose with increased insulin levels.
With much uncertainty remaining, it is probably a good idea to listen to our body and to how hungry we feel in response to different kinds of food.